We believe every discovery we make brings us one step closer to better treatments and potential cures. We offer interventional clinical trials to treat brain tumors and supportive care studies that explore how to increase the comfort and quality of life for individuals with brain cancer.
What Are Clinical Trials?
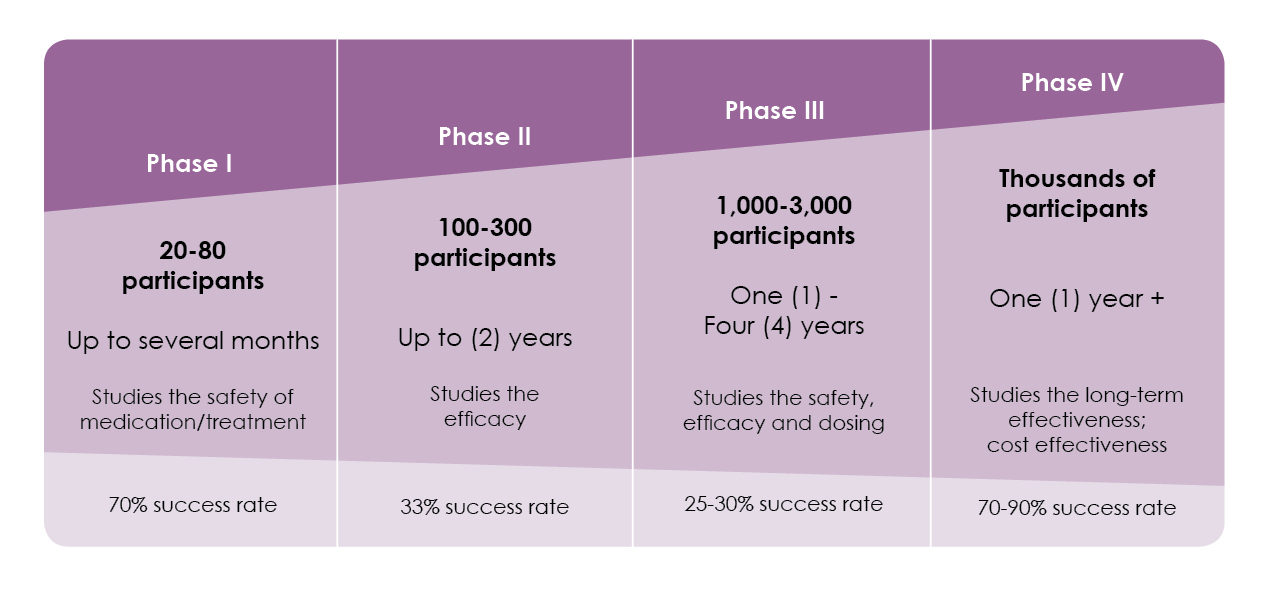
Clinical trials study new ways of treating patients. Taking part in a clinical trial is voluntary. If you choose not to participate, you will be offered the standard treatment for your health problem. You can talk to your health care provider if you have questions about clinical trials. There are different phases of clinical trials.
Who can join a clinical trial?
Talk to your team to find out if there are any studies they know of that might be right for your type of brain tumor.
Clinical trials follow a plan known as a protocol. The protocol describes who is eligible for each study. Each study must include only the people who meet the requirements for the study. Eligibility criteria are different for each study and include factors such as age, sex, type and grade of the brain tumor, previous treatment history, and whether you have other health problems.
The inclusion and exclusion criteria are not to reject anyone personally but to decrease the variation within the study and ensure that the researchers can answer their study questions.
What is informed consent?
Informed consent is the process of learning about the risks and benefits of treatment, asking questions about it, and deciding if it is right for you. Before starting your treatment, you get important information, including the risks and benefits of the treatment.
Read the informed consent and write down any questions you have. By signing the consent, you are saying that your team has told you about the risks and benefits of the treatment, you have asked all your questions, and we have answered them.