Overview
The Brown Lab studies mechanisms determining endogenous antitumor T cell priming and function in malignant brain tumors and other tumor types. In doing so, the lab seeks to develop new and improved in situ vaccine strategies and paradigms that engender productive immune rejection of tumors. To this end, we decode molecular mechanisms by which virotherapy induces antitumor T cell immunity, define mechanisms that improve the antitumor efficacy of in situ vaccination, and identify and mechanistically explain clinical correlates of response to immunotherapy.
Research Initiatives
Antitumor CD8 T cells rely upon several environmental cues to productively eliminate malignant cells, including within the tumor microenvironment and tumor draining lymph nodes. The overall goal of the Brown Lab is to mechanistically define signals within the tumor microenvironment and lymphatics to recruit and engage functional antitumor T cell responses.
Pattern Recognition Receptors (PRRs) recognize diverse pathogen (viruses, bacteria, fungi) features to induce innate immune inflammation. The innate arm of the immune system rapidly detects pathogens on the basis of ‘non-self’ features (e.g., double-stranded RNA) using PRRs and coordinate an anti-pathogen response that encompasses cytotoxic mediators, initiation of cell death, and inflammation that shapes and eventual adaptive immune response (Figure 1).
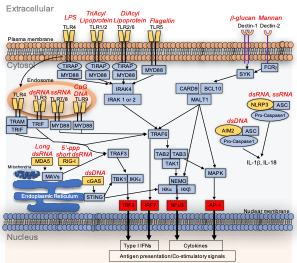
Figure 1. PRR cellular locations, specificities and downstream signaling. Yellow indicates PRRs, blue indicates signaling mediators, and red indicates transcription factors that mediate transcription of inflammatory genes. Arrows indicate signaling/modifications that transduce signals to tell the cell to induce inflammation. Pathogen associated features that activate PRRs are depicted in italicized red text. Upon recognizing a pathogen feature, PRRs induce signaling cascades that lead to the production of inflammatory mediators (including cytokines and type I IFNs), that lead to recruitment of additional immune cells and engage anti-pathogen immune responses.
PRRs control recognition and elimination of cancer cells by the immune system. PRR-induced inflammation is critical for a productive antitumor immune response (Figure 2).
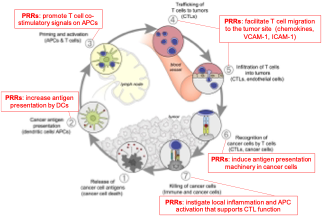
Figure 2. 1) Cancer cells routinely die due to stress, chemo/radiation, nutrient deprivation, hypoxia, etc; leading to the release of tumor antigens. 2) Migrating antigen presenting cells take up antigen in the tumor microenvironment to be processed and presented to T cells. PRRs potentiate this step by increasing antigen presentation, increasing antigen uptake, and recruiting additional migratory DCs into tumors. 3) Within the draining lymph node or other secondary lymphoid organ, tumor antigen loaded DCs present antigen to T cells, leading to their activation. PRR signaling induces the expression of co-stimulatory signals on DCs to potentiate T cell priming/activation. 4) T cells traffic to the site of the tumor by surveying PRR dependent endothelial ligand expression and chemokine signals, and 5) infiltrate the tumor tissue. 6) T cells recognize cognate tumor antigen presented on tumor cells; PRRs facilitate this process by inducing inflammation that causes induction of antigen presentation machinery in cancer cells. 7) T cells kill cancer cells expressing their cognate antigen via granzymes and perforin, FAS ligand, and secretion of cytotoxic cytokines; PRRs induce inflammation that enhances antitumor T cell function and cytotoxicity. Adapted from Chen and Mellman. Immunity 2013.
Triggering antiviral inflammation can revive antitumor immune responses, i.e. ‘in situ vaccination’. Our focus is to leverage the role of PRRs in the cancer immunity cycle (Figure 2) to mediate cancer immunotherapy (Figure 3). However, optimal routes to harness the innate immune system for cancer immunotherapy have yet to be clinically realized, molecular mechanisms by which the innate immune system controls antitumor immunity in gliomas are crudely defined, and determinants of in situ vaccination success remain unexplored.
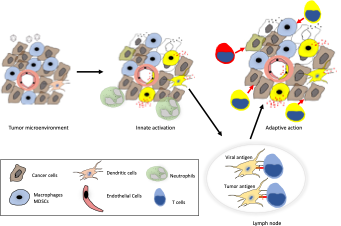
Figure 3. Intratumor administration of viruses and virus-like features engage PRRs in tumor associated cells, including macrophages which constitute up to 50% of the tumor mass in gliomas. The resultant innate inflammation can enhance tumor antigen presentation and delivery of activating signals to antitumor T cells. This in turn leads to generation of functional antitumor T cells that eliminate cancer cells and/or may prevent recurrence.
ONGOING RESEARCH INITIATIVES:
Leveraging CD4 T cell help for in situ cancer vaccination. We discovered that treating tumors with childhood vaccines, in particular polio and tetanus, mediates antitumor efficacy by coordinating antitumor CD8 T cells and eosinophils by re-activating (i.e., ‘recall’ of) vaccine-specific memory CD4 T cells (Brown et al JITC 2023). These studies reveal potential for childhood vaccines to accomplish in situ vaccination by enlisting local CD4 T cell help, and implies that Th2 polarized CD4 T cells and type II immune responses can positively influence cancer immune surveillance. Ongoing research is focused on optimizing ‘recall’ antigen density and character to maximize antitumor effects of CD4 T cell recall, resolving the role of eosinophils and type II immune mediators in cancer immune surveillance, as well as developing novel therapeutic strategies—including combining with PRR-engaging therapies— based upon these concepts.
Defining and optimizing antitumor T cell engagement after virotherapy and in situ vaccination. We discovered that type I IFNs are necessary, but not sufficient alone, to induce productive antitumor T cell activity in the context of in situ vaccination and virotherapy (Brown et al Nat Comm 2021). Our current work is resolving cellular, chemokine and cytokine determinants of successful in situ vaccination; as well as optimizing new PRR-engaging strategies that provoke Type I IFN contextualized innate inflammation commensurate with productive antitumor T cell engagement. Amongst these approaches include new delivery routes and strategies for polio virotherapy in collaboration with the Gromeier Lab, exploring the utility of peripheral and antitumor ‘priming’ of innate immunity prior to in situ vaccination, developing novel virotherapy candidates with broader type I IFN contextualized inflammatory footprints, as well as mechanistically explaining correlates of successful in situ vaccination in glioma patients.
Resolving targetable sex differences in brain tumor immune surveillance. In collaboration with Donald McDonnell’s research group, we are testing the role of sex hormones in influencing intratumor innate immunity, in particular in tumor associated myeloid cells, as a route to revive glioma immune surveillance. The goals of this project are to define sex differences and respective roles for sex hormone signaling in brain tumor immune surveillance, determine the role of sex hormone signaling in dictating glioma immune resistance, and to test if clinically available sex hormone modulators can be repurposed to target brain tumors.
Ex vivo glioma slice cultures as a pre-clinical model of anti-glioma drug discovery. Leveraging the strength of the Preston Robert Tisch Brain Tumor Biorepository, our team developed an ex vivo slice culture glioma model that differs from commonly used organoid and cell line techniques in that the native tumor microenvironment and architecture remains intact. We are using this model to define therapy potential of diverse in situ vaccine modalities, virotherapies, novel chemotherapy strategies, and combinations thereof to demonstrate mechanisms of action in human glioma tissue, identify biomarkers and mechanisms of resistance to therapy, and nominate new therapy strategies for gliomas.