
What is the purpose of this clinical trial?
The purpose is to study a new promising therapy called MVR-C5252 in patients with recurrent high grade glioma.
What is MVR-C5252?
MVR-C5252 is a herpes type 1 virus (cold sore virus) that has been modified to selectively infect cancer cells, secrete immune stimulating proteins, and train the patient’s own immune system to kill malignant cells. Aside from direct viral killing of cancer cells, the virus produces two proteins locally within the tumor, namely IL-12 and a PD1 inhibitor, that empowers killing of cancer cells by the immune system.
How is MVR-C5252 given to patients?
MVR-C5252 is given directly via a so called convection enhance delivery (CED) catheter (a thin plastic tube) into the tumor. The treatment is infused slowly over 4 hours.
What are the different stages of this trial?
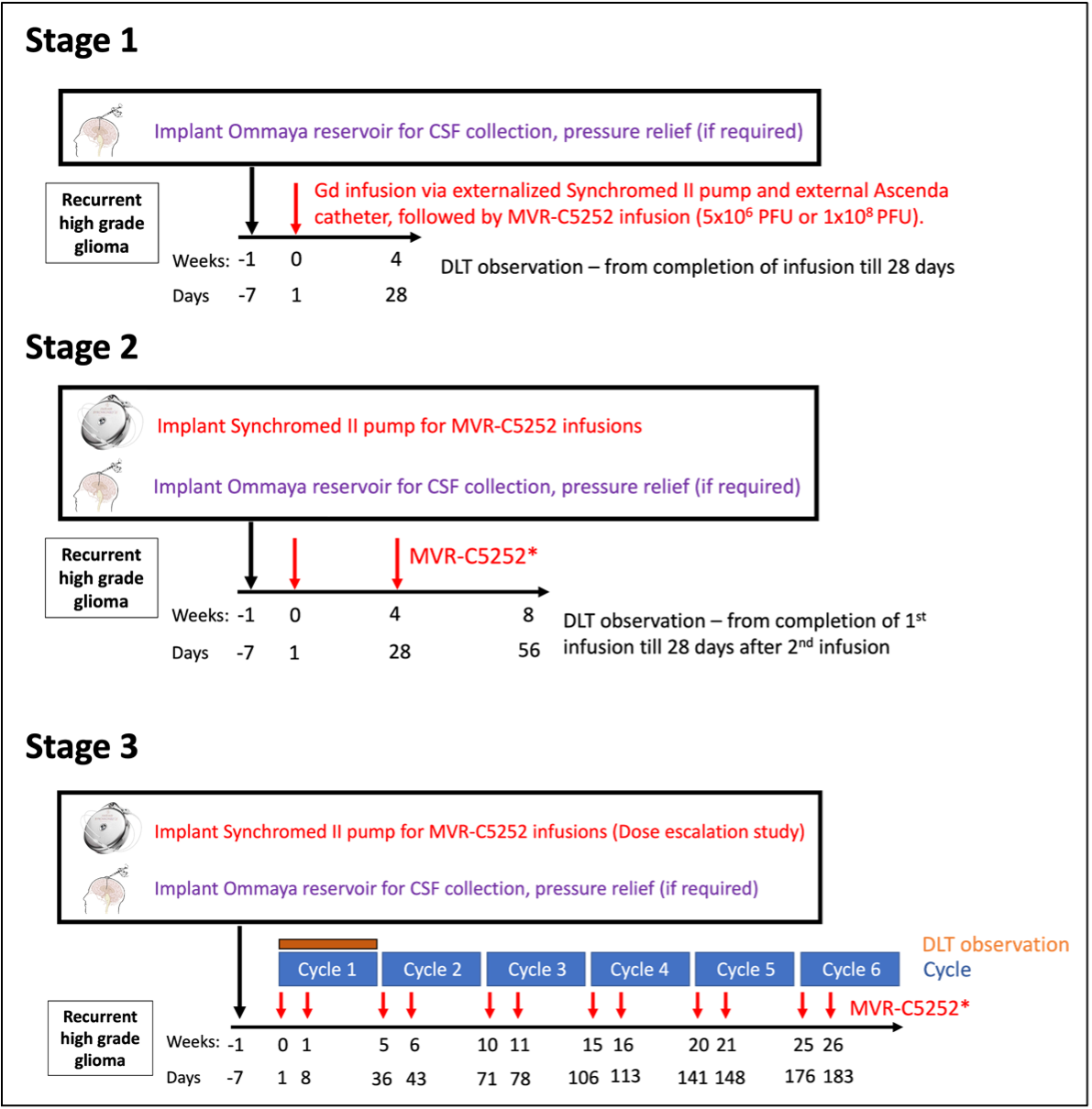
The trial will take place in multiple stages as follows:
Stage 1. Patients will have a biopsy to confirm that they have tumor recurrence. The study drug will be infused into the tumor via an external pump that is connected to an external catheter (a thin plastic tube). The tip of the catheter will be placed into the tumor at time of surgery. The first patient in stage 1 was treated on July 18th without any issues.
After determining safety and feasibility of a single injection, enrollment will proceed to stage 2.
Stage 2, Patients will receive 2 intratumor doses of the drug through an implantable (internalized) pump. The pump and catheter will be implanted underneath the skin. Two doses will occur 4 weeks apart. Once the safety has been established, we will proceed to stage 3.
Stage 3, Using the implantable pump, patients will receive the drug twice, 7 ± 1 day apart per cycle. Treatment cycles will continue until 12 infusions (6 cycles). Once the optimal dose of the drug has been established, we will proceed to Stage 4.
Stage 4, the dose expansion portion of the study, will commence once the optimal dose and schedule is established. Within Stage 4, the efficacy of MVR-C5252 will be evaluated.
What is the main thing researchers are looking for in this trial?
The main purpose of the trial is (1) to demonstrate that the treatment can be given to patients without major side effects (2) to demonstrate that the immune system is revived after treatment locally within the brain and (3) to demonstrate that the treatment helps patients by delaying the recurrence of the cancer and to help them live longer.
What does "open-label" mean in this trial?
Open label means that patients know what treatment they are receiving and that there is no placebo.
What is a "dose-limiting toxicity" (DLT)?
Dose limiting toxicity is any side effect that is considered problematic and unacceptable to patients.
How many times will patients get the treatment in Stage 2?
Twice
What is the Blood Brain Barrier (BBB), and why is it important in this trial?
It is the system that protects the brain from toxic chemicals, toxins and other harmful substances but this barrier also limits the ability of medical treatments that are taken by mouth or as an injection into the veins from arriving at the brain tumors from the blood stream. To circumvent the BBB, the drug will be infused directly into the tumor, ensuring the drug reaches and functions within the tumor.
What is the goal of Stage 4 of the trial?
Stage 4 will teach us how much benefit patients will receive in terms of delaying disease recurrence and prolonging their survival after an optimal dose is determined from stage 3.
Why are researchers using a virus to treat brain tumors?
Attenuated viruses cause the same type of immune responses needed for the immune system to recognize and kill cancer cells; they also selectively replicate in cancer cells (as opposed to normal cells or neurons). Due to the induction of antiviral immune responses, anti-tumor immune responses are also bolstered. This virus further facilitates that process by expressing immune stimulating proteins (IL-12 and PD1 blocking antibody).
What is progression-free survival?
Progression-free survival is the length of time during and after the treatment of a disease, such as cancer, that a patient lives with the disease, but it does not get worse.
How will researchers measure if the treatment is working?
By demonstrating that the immune system gets stronger against the cancer and that the treatment helps patients by delaying the recurrence of the cancer and to help them live longer.
What happens if the treatment causes bad side effects?
Affected patients will receive medical care and support to help them recover. If a treatment causes bad side effects, doctors immediately report the issues and may pause the trial to investigate. They might lower the drug dose, change the study plan to include more safety checks, or even stop the trial entirely if the side effects are severe.
To learn more about this trial or to contact the team regarding enrollment, please visit: https://www.dukehealth.org/clinical-trials/directory/pro00112883.